Chemistry, Department of: Faculty Series
Patrick Dussault Publications
Document Type
Article
Date of this Version
12-2010
Abstract
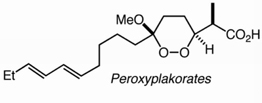
A convenient synthesis of sidechain-modified phytosterols is achieved via a temporary masking of the stigmasterol 5,6-alkene as an epoxide. Following performance of the desired modification, the alkene is regenerated through a mild deoxygenation. The approach is applied to the syntheses of β-sitosterol and campesterol acetate, and suggests a facile route to the (Z)-isomers of Δ22–23 phytosterols.
Includes Supplementary Data.


Comments
Published in Steroids 75:12 (December 2010), pp. 879–883; doi: 10.1016/j.steroids.2010.05.016 Copyright © 2010 Elsevier Inc. Used by permission