Chemistry, Department of: Faculty Series
Patrick Dussault Publications
Document Type
Article
Date of this Version
2009
Abstract
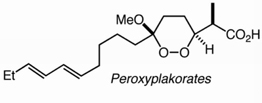
The stereospecific intramolecular alkylation of a hydroperoxyacetal provides the basis for the first asymmetric synthesis of the dioxane propionate core of the peroxyplakorates. Chemoselective hydrometallation of an alkyne in the presence of a peroxide is used to introduce a synthon for the polyunsaturated side chains of the peroxyplakorates. The route suggests a general solution for the 1,2-dioxane unit in many peroxide natural products.


Comments
Published in Tetrahedron 65 (2009), pp. 9680–9685; doi:10.1016/j.tet.2009.09.068 Copyright © 2009 Elsevier Ltd. Used by permission.