Chemistry, Department of
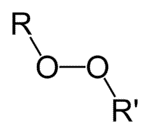
Organic Peroxides: Safety Issues
Date of this Version
7-31-2018
Document Type
Article
Citation
Department of Chemistry, University of Nebraska-Lincoln
doi:10.13014/K2GT5KCJAbstract
ABSTRACT: The following is a brief introduction to peroxide safety from the viewpoint of a synthetic chemist. Major topics include: classes of organic peroxides; peroxide reactivity; hazard identification and minimization; reaction monitoring; and, hazards associated with auto-oxidation of peroxidizable solvents.
KEYWORDS: peroxide, hydroperoxide, hydroperoxyacetal, ozonide, perester, dihydroperoxide, monoperoxyacetal, active oxygen, peroxide strips, TLC indicator, self-accelerating decomposition, radical, exothermicity, transition metals.
CONTENTS: 1. Literature resources 2. Major classes of peroxides 3. Basis for peroxide reactivity 4. Determining reactivity and minimizing hazards a. Steps to take before an experiment b. Minimizing hazard during reactions c. Monitoring reactions d. Working with isolated peroxides 5. Peroxide toxicity 6. Peroxidizable solvents and reagents 7. Guidelines for handling and transport


Comments
Copyright (c) 2016 Pat Dussault. Revised June 2017 & July 2018.