Chemistry, Department of: Faculty Series
Robert Powers Publications
Document Type
Article
Date of this Version
1992
Citation
Biochemistry 1992, 31, 4347-4353
Abstract
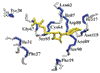
The secondary structure of human recombinant interleukin-4 (IL-4) has been investigated by three-dimensional (3D) 15N- and 13C-edited nuclear Overhauser (NOE) spectroscopy on the basis of the 1H, 15N, and 13C assignments presented in the preceding paper [Powers, R., Garrett, D. S., March, C. J., Frieden, E. A,, Gronenborn, A. M., & Clore, G. M. (1992) Biochemistry (preceding paper in this issue)]. Based on the NOE data involving the NH, CαH, and CβH protons, as well as 3JHNα coupling constant, amide exchange, and 13Cα and 13Cβ secondary chemical shift data, it is shown that IL-4 consists of four long helices (residues 9-21, 45-64, 74-96, and 113-129), two small helical turns (residues 27-29 and 67-70), and a mini antiparallel β-sheet (residues 32-34 and 110-1 12). In addition, the topological arrangement of the helices and the global fold could be readily deduced from a number of long-range interhelical NOEs identified in the 3D 13C-edited NOE spectrum in combination with the spatial restrictions imposed by three disulfide bridges. These data indicate that the helices of interleukin-4 are arranged in a left-handed four-helix bundle with two overhand connections.


Comments
U.S. Government Work