Chemistry, Department of: Faculty Series
John J. Stezowski Publications
Document Type
Article
Date of this Version
April 2002
Abstract
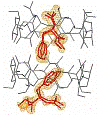
In a systematic study of molecular recognition of amino acid derivatives in solid-state β-cyclodextrin (β-CD) complexes, we have determined crystal structures for complexes of β-cyclodextrin/N -acetyl-L-phenylalanine at 298 and 20 K and for N-acetyl-D-phenylalanine at 298 K. The crystal structures for the N-acetyl-L-phenylalanine complex present disordered inclusion complexes for which the distribution of guest molecules at room temperature is not resolvable; however, they can be located with considerable confidence at low temperature. In contrast, the complex with N-acetyl-D-phenylalanine is well ordered at room temperature. The latter complex presents an example of a complex in this series in which a water molecule is included deeply in the hydrophobic torus of the extended dimer host. In an effort to understand the mechanisms of molecular recognition giving rise to the dramatic differences in crystallographic order in these crystal structures, we have examined the intermolecular interactions in detail and have examined insertion of the enantiomer of the D-complex into the chiral β-CD complex crystal lattice.


Comments
Published in Proceedings of the National Academy of Sciences of the United States of America 99:8 (April 16, 2002), pp. 5115-5120. doi: 10.1073/pnas.072647599 Copyright © 2002 National Academy of Sciences. Used by permission.