Department of Physics and Astronomy: Publications and Other Research
Ilya Fabrikant Publications
Document Type
Article
Date of this Version
2008
Abstract
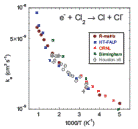
The validity of the Arrhenius equation for dissociative electron attachment rate coefficients is investigated. A general analysis allows us to obtain estimates of the upper temperature bound for the range of validity of the Arrhenius equation in the endothermic case and both lower and upper bounds in the exothermic case with a reaction barrier. The results of the general discussion are illustrated by numerical examples whereby the rate coefficient, as a function of temperature for dissociative electron attachment, is calculated using the resonance R-matrix theory. In the endothermic case, the activation energy in the Arrhenius equation is close to the threshold energy, whereas in the case of exothermic reactions with an intermediate barrier, the activation energy is found to be substantially lower than the barrier height.


Comments
Published in THE JOURNAL OF CHEMICAL PHYSICS 128, 124308 (2008). Copyright © 2008 American Institute of Physics. Used by permission.