Chemistry, Department of: Faculty Series
James M. Takacs Publications
Document Type
Article
Date of this Version
2-12-2006
Abstract
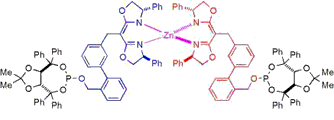
The chirality-directed self-assembly of bifunctional subunits around a structural metal-typically, zinc(II)-is used to form a heteroleptic complex in which a second set of ligating groups are suitably disposed to bind a second metal, forming a heterobimetallic catalyst system. We find that subtle changes in the structural backbone (i.e., ligand scaffold) of such chiral bidentate self-assembled ligands (SALs) can be used to manipulate the ligand topography and chiral environment around catalytic metal; thus, the scaffold can be optimized to maximize asymmetric induction. Using this combinatorial strategy for ligand synthesis, a preliminary study was carried out in which a library of 110 SALs was evaluated in the rhodium-catalyzed asymmetric hydrogenation of a simple N-acyl enamide. The level of enantioselectivity obtained varies from near racemic to greater than 80% ee as a function of the ligand scaffold, with the possibility of further improvement yet to be explored.


Comments
Published in Pure and Applied Chemistry 78:2 (February 2006), pp. 501-509. Copyright © 2006 International Union of Pure and Applied Chemistry. Used by permission.
http://www.iupac.org/publications/pac/