Chemistry, Department of: Faculty Series
James M. Takacs Publications
Document Type
Article
Date of this Version
December 2007
Abstract
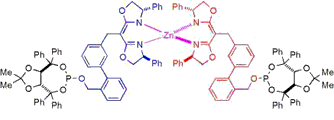
A self-assembled ligand library (SAL XY) affords a wide range of R/S ratios in Rh-catalyzed asymmetric hydroboration (nbd = 2,5norbornadiene, R* is a chiral substituent). Ligand-scaffold optimization reveals “substrate-tailored” ligands that afford high regio- and enantioselectivity for a variety of ortho-substituted styrene derivatives.
Includes article (4 pp.) and supporting information (106 pp.).


Comments
Published in Angewandte Chemie Int. Ed. 46 (2007); doi: 10.1002/anie.200703127 Online at http://www3.interscience.wiley.com/cgi-bin/abstract/117870359/ABSTRACT Copyright © 2007 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Used by permission.