Chemistry, Department of: Faculty Series
Stephen DiMagno Papers
Document Type
Article
Date of this Version
2011
Abstract
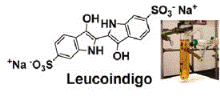
Catalytic, low temperature preferential oxidation (PROX) of carbon monoxide by aqueous [5,10,15,20-tetrakis- (4-sulfonatophenyl)-2,3,7,8,12,13,17,18-octafluoroporphyrinato]- rhodium(III) tetrasodium salt, (1[Rh(III)]) and [5,10,15, 20-tetrakis(3-sulfonato-2,6-difluorophenyl)-2,3,7,8,12,13, 17,18-octafluoroporphyrinato]rhodium(III) tetrasodium salt, (2[Rh(III)]) is reported. The PROX reaction occurs at ambient temperature in buffered (4 ≤ pH ≤ 13) aqueous solutions. Fluorination on the porphyrin periphery is shown to increase the CO PROX reaction rate, shift the metal centered redox potentials, and acidify ligated water molecules. Most importantly, β-fluorination increases the acidity of the rhodium hydride complex (pKa = 2.2 ± 0.2 for 2[Rh-D]); the dramatically increased acidity of the Rh(III) hydride complex precludes proton reduction and hydrogen activation near neutral pH, thereby permitting oxidation of CO to be unaffected by the presence of H2. This new fluorinated water-soluble rhodium porphyrin-based homogeneous catalyst systempermits preferential oxidation of carbonmonoxide in hydrogen gas streams at 308 K using dioxygen or a sacrificial electron acceptor (indigo carmine) as the terminal oxidant.


Comments
Published in ACS CATALYSIS Volume: 1 Issue: 7 Special Issue: SI Pages: 764-771 DOI: 10.1021/cs2001187
This article is a U.S. government work, and is not subject to copyright in the United States.