Chemistry, Department of: Faculty Series
Robert Powers Publications
Document Type
Article
Date of this Version
1993
Citation
Journal of Magnetic Resonance, Series B 101, 325-327 (1993)
Abstract
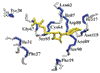
One of the principal motivations for studying proteins by nuclear magnetic resonance stems from the desire to describe the solution structure of these molecules as compared to the generally perceived static picture obtained by X-ray crystallography. Indeed, it is one of the unique features of NMR spectroscopy that in addition to structural data, dynamic properties can be probed and characterized by measuring relaxation parameters. Furthermore, any mobility of the protein in solution will necessarily modulate the measured NMR parameters and should influence the resulting structure. It has been argued that regions of a protein that are highly mobile would be expected to be defined to a lesser degree of precision than regions that are rigid (1. 2 ).


Comments
U.S. Government Work