Chemistry, Department of: Faculty Series
Andrzej Rajca Publications
Document Type
Article
Date of this Version
April 2001
Abstract
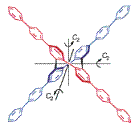
The asymmetric synthesis of a chiral, nonracemic π-conjugated system with D2 point group of symmetry, dodecaphenylene 4 is described. In the key step, (−)-sparteine- and Cu(II)-mediated oxidation of 2,2′-dilithio-1,1′-biaryls in ether gives the corresponding dimers, tetra-o-phenylenes, in 80% isolated yields and 50–60% ee’s. X-Ray crystallography confirms the structure of rac-4 and its molecular shape of a Greek cross. The torsional angles between the benzene rings in the tetra-o-phenylene core of rac-4 are in the 56.5–71.0° range. However, CD and UV spectra of 4 in CH2Cl2 are consistent with significant conjugation between the four terphenyl moieties.


Comments
Published in Tetrahedron 57:17 (April 23, 2001), pp. 3725–3735. doi:10.1016/S0040-4020(01)00241-1 http://www.sciencedirect.com/science/journal/00404020 Copyright © 2001 Elsevier Science Ltd. Used by permission.