Plant Pathology, Department of
Department of Plant Pathology: Faculty Publications
Document Type
Article
Date of this Version
May 2005
Abstract
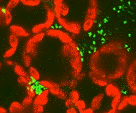
Prasher (42) cloned a cDNA for the green fluorescent protein (GFP) gene from the jellyfish Aequorea victoria in 1992. Shortly thereafter, to the amazement of many investigators, this gene or derivatives thereof were successfully expressed and conferred fluorescence to bacteria and Caenorhabditis elegans cells in culture (10, 31), followed by yeast (24, 39), mammals (40), Drosophila (66), Dictyostelium (23, 30), plants (28, 49), and filamentous fungi (54). The tremendous success of GFP as a reporter can be attributed to unique qualities of this 238- amino-acid, 27-kDa protein which absorbs light at maxima of 395 and 475 nm and emits light at a maximum of 508 nm. The fluorescence of GFP requires only UV or blue light and oxygen, and therefore, unlike the case with other reporters (β-glucuronidase, β-galacturonidase, chloramphenicol acetyltransferase, and firefly luciferase) that rely on cofactors or substrates for activity, in vivo observation of gfp expression is possible with individual cells, with cell populations, or in whole organisms interacting with symbionts or environments in real time. Complications caused by destructive sampling, cell permeablization for substrates, or leakage of products do not occur. Furthermore, the GFP protein is extremely stable in vivo and has been fused to the C or N terminus of many cellular and extracellular proteins without a loss of activity, thereby permitting the tagging of proteins for gene regulation analysis, protein localization, or specific organelle labeling. The mature protein resists many proteases and is stable up to 65°C and at pH 5 to 11, in 1% sodium dodecyl sulfate or 6 M guanidinium chloride (reviewed in references 17 and 67), and in tissue fixed with formaldehyde, methanol, or glutaraldehyde. However, GFP loses fluorescence in methanol-acetic acid (3:1) and can be masked by autofluorescent aldehyde groups in tissue fixed with glutaraldehyde. Fluorescence is optimal at pH 7.2 to 8.0 (67).


Comments
Published in APPLIED AND ENVIRONMENTAL MICROBIOLOGY, May 2001, p. 1987–1994 Vol. 67, No. 5. Copyright © 2001, American Society for Microbiology. Used by permission.