Plant Pathology, Department of
Department of Plant Pathology: Faculty Publications
Document Type
Article
Date of this Version
2011
Citation
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Jan. 2011, p. 416–426
Abstract
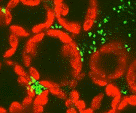
Surfaces made of copper or its alloys have strong antimicrobial properties against a wide variety of microorganisms. However, the molecular mode of action responsible for the antimicrobial efficacy of metallic copper is not known. Here, we show that dry copper surfaces inactivate Candida albicans and Saccharomyces cerevisiae within minutes in a process called contact-mediated killing. Cellular copper ion homeostasis systems influenced the kinetics of contact-mediated killing in both organisms. Deregulated copper ion uptake through a hyperactive S. cerevisiae Ctr1p (ScCtr1p) copper uptake transporter in Saccharomyces resulted in faster inactivation of mutant cells than of wild-type cells. Similarly, lack of the C. albicans Crp1p (CaCrp1p) copper-efflux P-type ATPase or the metallothionein CaCup1p caused more-rapid killing of Candida mutant cells than of wild-type cells. Candida and Saccharomyces took up large quantities of copper ions as soon as they were in contact with copper surfaces, as indicated by inductively coupled plasma mass spectroscopy (ICP-MS) analysis and by the intracellular copper ion-reporting dye coppersensor-1. Exposure to metallic copper did not cause lethality through genotoxicity, deleterious action on a cell’s genetic material, as indicated by a mutation assay with Saccharomyces. Instead, toxicity mediated by metallic copper surfaces targeted membranes in both yeast species. With the use of Live/Dead staining, onset of rapid and extensive cytoplasmic membrane damage was observed in cells from copper surfaces. Fluorescence microscopy using the indicator dye DiSBaC2(3) indicated that cell membranes were depolarized. Also, during contact-mediated killing, vacuoles first became enlarged and then disappeared from the cells. Lastly, in metallic copper-stressed yeasts, oxidative stress in the cytoplasm and in mitochondria was elevated.


Comments
Copyright © 2011, American Society for Microbiology.
doi:10.1128/AEM.01704-10